First in Class Therapies to Prevent Epilepsy
Mission
We aim to develop treatments that prevent epilepsy after brain injuries such as head trauma, stroke and infection. Acquired seizures after such causes comprise at least 20% of all epilepsy, a disease that affects 1% of the world population. Our goals are to protect people from developing epilepsy and to spare them from its devastating consequences.
About PrevEp
PrevEp is a US-based biotech company founded by world leaders in epilepsy research with decades of combined experience in the development of new therapies for epilepsy prevention. The founders combine deep expertise in basic science, drug development and clinical trials in epilepsy after CNS injury. The intimate collaboration between preclinical and clinical team members enables PrevEp to rapidly translate preclinical discoveries to clinical trials.
Our Strategy
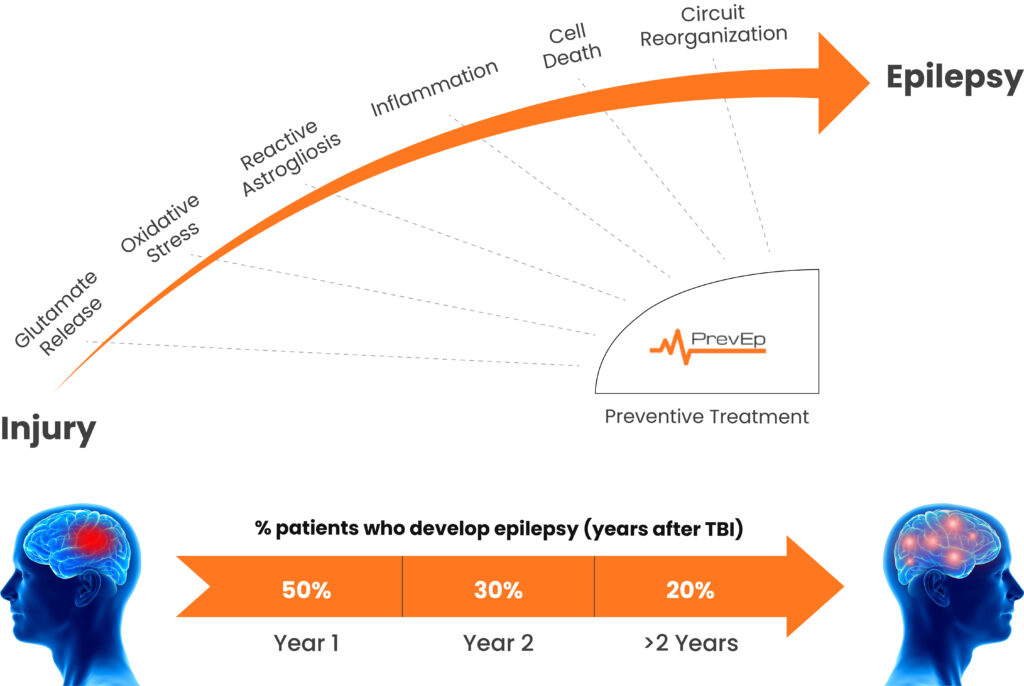
Epileptogenesis is a complex process that is unlikely to be arrested by a single drug. PrevEp leverages recent neuroscience discoveries in network pharmacology, and availability of proven and safe molecules to design combination therapies that target a multitude of epileptogenesis targets, in the earliest stages after brain injury. Our lead combinations are well-tolerated and have proven to block epileptogenesis in a predictive rodent model.
Our Team

Pavel Klein
President co-founder
Pavel Klein, M.D. has >20 years clinical epilepsy research experience. Dr. Klein is PI of a recent NIH-funded clinical PTE prevention study, and current DoD PTE grant PI involving a consortium of 10 US and European trauma/epilepsy centers

Wolfgang Löscher
Chief Scientific Officer co-founder
Wolfgang Löscher, D.VM., Ph.D. has >40 years translational epilepsy research experience. He is a highly decorated epilepsy scientist with extensive academic and industry experience in drug development. He discovered the unique preclinical profile of levetiracetam that initiated its development for treatment of epilepsy, resulting in levetiracetam becoming the globally most used anti-seizure medication (ASM). Dr Löscher is one of 6 most widely cited epilepsy researchers in the world, with unmatched record in preclinical epilepsy drug development.

Chris Rundfeldt
Vice President, Toxicology and Regulatory Affairs co-founder
Chris Rundfeldt, D.VM., Ph.D. has >30 years epilepsy and drug development research experience in pharmaceutical industry. Dr Rundfeldt discovered another antiseizure medication, retigabine/ezogabine (EU)/US), with a unique new target mechanism of action, initiating its development, approval and clinical use. Dr. Rundfeldt’s identification of the KCNQ/KV7 channel opening properties of this molecule not only facilitated the development of retigabine/ezogabine, but also triggered the development of second and third generations of new drugs, KCNQKV7 channel modulators, in epilepsy, pain and urinary incontinence.Dr. Rundfeldt subsequently became independent drug development expert. He supported, among other companies, the danish biotechstart up, Forward Pharma AS, in their successful IPO at NYSE.

Detlev Boison
Chief Development Officer co-founder
Detlev Boison, Ph.D. has >25 years translational epilepsy research experience. He is the PI of active antiepileptogenic drug discovery program, and identified a novel molecular target for antiepileptogenic therapy.

Alexander Rotenberg
Biomarker Development co-founder
Alexander Rotenberg, M.D., Ph.D., Professor of Neurology (pediatric epilepsy) at Harvard Medical School, has >15 years of translational and clinical epilepsy experience. He is a leading basic researcher in preclinical post-traumatic epilepsy mechanisms and treatments, and a world leading clinician researcher in the use of transcranial magnetic stimulation (TMS) as a biomarker of brain excitability used in human antiseizure medication proof of concept study testing. He has been the PI of recent DoD and NIH PTE grants, and has discovered an anti-epileptogenic target and related biomarker that are incorporated into the PrevEp portfolio and strategy.

Alexander Kraus
Chief Business Officer
Alexander Kraus, Ph.D. is an international pharmaceutical executive with more than 20 years of experience in branded pharmaceutical industry. Dr. Kraus provides broad experience in R&D, business and portfolio development, strategic communications, and management competence in various therapeutic areas and indications

Matthias Koepp
Vice President, Product Devel. co-founder
Matthias Koepp, M.D. has >30 years experience as a highly-cited and widely-published expert in epilepsy therapeutic product development. He has co-directed a recent, ongoing trial of prevention of epilepsy after stroke, the first Phase 3 study of prevention of post-stroke epilepsy ever done, the clinical design of which was based on his co-discovery of predictors of post-stroke epilepsy.
Advisory Board
Our company concept is supported by leading epilepsy ambassadors
Jacqueline A. French, M.D.
Professor of Neurology and Director of clinical trials at NYU Langone‘s Comprehensive Epilepsy Center; Chief Scientific Officer, Epilepsy Foundation
Michael A. Rogawski, M.D., PhD.
Professor of Neurology and Pharmacology, Director of Institute for Neurotherapeutics, University of California, Davis
Elisabeth Garofalo, M.D.
Previous Senior VP, Novartis Pharmaceuticals
Annamaria Vezzani, Ph.D.
Professor of Neuroscience, Mario Negri Institute, Milan, Italy
Henrik Klitgaard, Ph.D.
Previous Vice President, Neurosciences Therapeutic Area, UCB Biopharma
Enrique Carrazana, M.D.
Chief Medical Officer, Neurelis
Meir Bialer, Ph.D., MBA
Professor Emeritus of Pharmacy, The Hebrew University, Jerusalem, Israel


